In order to work as intended, this site stores cookies on your device. Accepting improves our site and provides you with personalized service.
Click here to learn more
Click here to learn more
With the March congress on the horizon, we take a look at some of the trends within the industry over the last year, and what to expect from the March event.
It’s not a surprise that, with the impact of the pandemic, the importance of digitisation has been heavily reinforced. In early 2020, we reflected on Scimcon’s experience of providing remote support to clients and some of the changes we witnessed as a result of the COVID-19 pandemic, and now almost two years on, we’re seeing a new way of working across labs and organisations.
With digital transformation hot on the global agenda, what’s next for analytical and clinical laboratories? What will the lab of the future look like? Lab of the Future’s March congress aims to answer that question.
With a selection of activities scheduled across the 2-day event, there is no shortage of opportunity for attendees to get involved – whether that’s in-person in the Boston, MA event, or from the comfort of their own workspace via virtual attendance.
The agenda features a range of roundtables and presentations, including plenary sessions, as well as more focussed discussions on specific topics, from the digital lab to the connected innovation lab. The tradeshow will also feature plenty of networking session throughout, allowing individuals to form valuable new connections and learn more about some of the key players and innovation across the industry.
The event also welcomes a wide of speakers presenting and hosting discussions during the 2-day period. With confirmed speakers from GSK, Merck, Pfizer, Boehringer Ingelheim, and Astrazeneca, amongst many others, it’s guaranteed to be an event filled with interesting discussions from some of the organisations that have become household names over the last 24 months.
In addition to discussions, the event is also hosting technology showcases, for leading solution providers to demonstrate some of the latest and most disruptive innovation that they’ve been perfecting behind the scenes. Focussed work tracks also allow attendees to take a more in-depth look at some of the latest technologies and trends in 4 key areas – lab automation, digitalisation, connectivity, and innovation.
Lab of the Future is an insightful event, and one that we look forward to as well as sponsor each year. The in-person aspect of the event will make for a refreshing change following the pandemic restrictions experienced worldwide, but the additional virtual element of the tradeshow means that users around the globe can participate and get involved, regardless of restrictions and concerns surrounding COVID-19 and travel.
However, in addition to the event, the lab of the future is a concept. Our team at Scimcon has over 20 years of experience in laboratory informatics, and with many of our team members having direct lab experience, we can help you get your digitisation and laboratory informatics project off the ground, whilst understanding the questions and concerns faced by scientists every day.
I didn’t really fit into the company I was in before, so firstly, I think to enjoy any role the people you work with are important. Realistically, I probably spend more time speaking with my colleagues than I do my own wife and kids, so having great colleagues definitely makes Scimcon enjoyable.
Secondly, the challenges within the role are exciting. Whenever you start a new contract – as we do regularly, supporting clients in pharma and biopharma – there is either an implementation project that needs carrying out, or an issue that needs resolving. Sometimes, the task at hand is not something I am directly familiar with or have the exact experience doing, but that makes it a challenge. And it’s the challenge that I enjoy.
In my prior role, we partnered with Scimcon on a number of projects. I had been involved in building a platform for our mutual client and when they needed to take the project forward, they needed someone to train, support, and project manage the implementation of the project software. It seemed a no-brainer for me to join the consultancy team at Scimcon, and to continue to support the project and the client to help them to progress. The transition into Scimcon was seamless, and I later moved from that initial project within the vaccines space onto a biotech company in the Netherlands, whom I have been supporting on e-systems.
My background helps me to bring people and team skills into play, so I am very suited to the Scimcon way of working, to support clients and to manage processes, SOPs, digital, and software projects for scientific companies. The client I am supporting currently was initially reluctant to appoint a lab informatics consultancy that was not local, and questions were raised as to whether or not we could perform the role from the UK without impacting the level of support we provide. As a test run, we were initially only working on a 3-month contract. Their processes are crucial to the business scale-up and growth, and our experience with other big pharma organisations has come into play helping them to navigate the decisions needed. I think we successfully demonstrated to them that it is feasible to be productive offering great support from the UK – so much so that the original 3-month project is now 30 months.
Every child has a hobby growing up, and of course in England, playing sports is probably one of the most popular activities. For me, football was my favourite pastime which evolved into more than just a hobby. It actually paved a pathway for all areas of my life.
What started out as a recreational game became a profession, as I left school at 16 to become a full-time football player, At 20, when my professional playing career finished, football again opened a window of opportunity to move to Southern California and coach football for 3 months. I returned 12 years later!
At 27, football again paid for my University education at Point Loma Nazarene University in San Diego, where I was sponsored via a soccer scholarship to study Business and Finance. I had never even enjoyed school, never mind imagined studying at University, but football provided a unique opportunity that was too good to refuse.
During my senior year at University, while coaching football in Denver during the summer, I was introduced to my fiancé Sarah who had also – coincidentally – attended University in the USA on a football scholarship. She was almost in a parallel to me, only she was based in Florida while I was on the total opposite side of the country in California.
Sarah and I had our first child then returned to the UK shortly after, where we now have four sons – aged 10, 7, 5, and 1. Our 10 and 7-year-olds are, like their mum and dad, football-mad, so we spend our weekends travelling to watch them play. Our 5-year-old is starting to get the bug, and we are yet to see if football is something our youngest warms to, but I can’t see the apple falling too far from the tree.
I still spend every day at some sort of football activity. Both our older boys are at the football academies so they each have 3-4 days a week training and playing. Football remains our outside-of-work life, having brought us together all those years ago.
And with the skills I learned at university, studying business and finance, as well as the team skills associated with playing sport, I am well placed to bring team leadership to the Scimcon family, and to focus on the best tactics and teams to work on a winning side.
Southern California was my home for 12 years, and having attended University in San Diego, that would be my obvious choice. We have a lot of friends and happy memories there, and we love the area – and the food!
We made the decision to return to UK as a family when we had the boys, for the support network of close family and friends. As I have been working from home for so many years anyway, personally my life hardly changed. However, it was a terrible impact for the boys. For any young kids, that transitional age and loss of companionship has been a huge negative experience. Luckily, the boys are pretty resilient and seemed to have bounced back into the new normal without any issues.
Funnily enough, I am not really a techie! I use a PC and a phone, but not much else.
Even though I don’t spend a great deal of time on gadgets and gizmos, I love the knowledge and benefits you can see from technology. For example, having spent so many of my formative years playing and coaching football, I am now still involved with my sons and their training and I see the technology to hand, which was never available when I was playing in the US. It’s absolutely fascinating, and there’s two pieces of tech in particular that I’m seeing used regularly at my sons’ training and matches. One is a Veo sports camera, which follows the ball and records the play, the other is an APEX GPS tag. This handy piece of kit is worn in the back of my son’s shirt when he’s playing, and all the data collected throughout the match is recorded on a smartphone, recording metrics such as speed, average position, and provides an overall performance review.
It’s amazing to see two of my previously completely separate worlds – football and technology – coming together, and it is really interesting to see how this technology is enabling football. As a coach, it also allows me to see and read the data available, so that we can then determine what is needed to improve in a game.
Scimcon is not a technology company, it is a people company that helps to solve the technology challenges for our clients, whether they be implementation, process or project-related. I am happy that I am not a techie, but very much a team and a people-person who can always learn and bring new skills to the table.
Podcast: Scimcon discusses digital transformation?With 20 years’ experience in the Biotech and Life Sciences industry, Micah Rimer’s success has been primarily due to his ability to read organisations, frame the problems and identify the best path to bring people together to achieve the desired goals. During Micah’s time working in Biotech and at big pharma he has successfully deployed consultancy groups within lab informatics and clinical projects.
Scimcon has supported Micah and his teams extensively on high profile projects in big pharma and Biotech. In this blog, Micah draws on his valuable experience to provide insight and tips on how to best to engage and work with consultancy groups.
Deciding on the right Informatics Consultant for your pharma or Biotech company is not a simple task, despite the various vendors available. Informatics in the Life Sciences industry covers a wide scope, so it is unlikely you will find an appropriate consultant through Google’s search algorithms. To find the best fit, you should consider what the key priorities are for your engagement, find a partner that you can trust and work well with and one that can bring a unique perspective to your collaboration.
Starting out with the key skills and contributions that are needed, as in any selection process, is the first step in identifying the right Informatics Consultant.
While technology is constantly advancing, I would encourage you to think hard if the tech skills are really the most important aspect for a successful collaboration. From my experience, while an understanding of the underlying technology and what it is capable of is important (be that LIMS, ELN, eCOA, eDiaries, PV software etc), what is of more importance is someone who can both communicate and partner with the organisation. A successful computerised system consists not just of the software, but also the people that will use it and the processes they will follow. All of those components must be balanced for a successful implementation, so while the technology piece may feel like the most obvious area to address, do not underestimate the work with the people and processes.
For example, there was a point in my career where I was asked to implement a Pharmacovigilance (PV) signal detection system for an organisation I was employed by. This had been a critical gap in the organisation for a long period of time, and there were several options on how to proceed, but no easily defined right answer. We could look to either evaluate and buy something off the shelf, hire a company to build a custom-designed tool, or alternately we could try to finalise a prototype that a programmer in the department had been playing around with for a while. (His tool had nice features, but still some technical gaps and no clear path forward to make it robust enough to use in such a highly regulated environment). In looking for external help, some may have favoured looking to recruit Informatics Consultants with a background in Pharmacovigilance, or perhaps with the technology skills to leverage the drug safety system platform. But in choosing Scimcon, I went with the partner I trusted to help evaluate the options and lead a successful implementation in that particular organisation. A fantastic off-the-shelf tool would never have been a success if people did not want to use it because they preferred the home grown highly customized (but invalidated) prototype.
With Scimcon on board, we were able to evaluate the pharma and drug safety landscape and determined that there could be a good path forward with the prototype that had already been developed. We were able to establish an effective team, drawing upon the Pharmacovigilance expertise in the department to address business process and usage questions. The programmer who built the prototype had the vision for how the software should work and what needed to be done from a technical perspective. Scimcon was able provide the knowledge and experience of how to move a prototype to a production system and validate a custom-defined tool that had been built by a programmer who does not have expertise in documentation. The output was widely recognised as a huge success.
It is important to keep in mind what gaps you are trying to close and what capabilities are needed to fill them: – Maybe you need the knowhow to get a project completed in a challenging environment; the technical skills to do the programming; the expertise with documentation; or you need to access people who have exceptional attention to detail to make sure a system is appropriately qualified and works as intended.
Whatever your challenge, you should ensure that the skillsets available from your informatics consultancy match the challenges you are facing.
As with many relationships, the more time you work together, the more trust you build up. This just proves that the importance of having established relationships and ways of working cannot be understated. No one is perfect, but the devil you know may indeed be a better fit than someone new to you. Always think about building up relationships for the future; it is a small world.
With this in mind, when looking for life science Informatics Consultants to partner with, one key consideration is to determine how they will be able to deal with adversity. What are they prepared to do if they see that a project is starting to go sideways? When speaking with them, ask them about projects that did not go according to plan or did not work out, and how did they manage that situation? What did they learn from that? Do they do anything differently from those lessons learned? Consultants, and Informatics Consultants especially, have all been thrown into projects where the requirements and expectations were not appropriately set in the beginning, which lead to problems later. Good consultants always learn from these situations and avoid repeating the mistakes that led them down that path.
Another key consideration for the interviewing and recruitment process is the quality of the questions the consultants ask and how well they listen.
Before you speak with them, think about what questions you would ask if you were in their shoes, and not having access to all the internal information you have: – Do they ask the right questions? Maybe they ask some questions you had not thought of? And how well are they able to play back what you have told them?
Often sales or account managers are very focused on telling you how easy it is for their teams to deliver, and how they will be able to deliver no matter what restrictions and conditions you add into the situation. Are there any conditions of the setup that would prevent the consultants from accepting the assignment? If there is nothing you can state that would cause them to be concerned, then maybe they are too good to be true! Look for their understanding that implementing systems is not usually a walk in the park. A truthful consultant is extraordinarily valuable.
Of course, it is always good to check the references as well. Does the life science informatics consultant have customers that have worked with them over a long period of time at different companies?
Bringing in consultants is typically not so easy, so numerous long-term engagements at different stops can itself also be a sign of delivering quality.
You should also look for feedback on the work the consultants delivered. There is a huge business out there for the larger agencies which spend time and resources selling at the executive level, but then use more junior resources to do the work. Having senior people presenting and being able to provide a concise message is important, but by and large you typically want to find people who are getting the work done. You must never lose sight of the goals to deliver projects on time and the overall drive for results.
Consider all of these aspects and listen to your instincts. Typically, these are not small or unimportant investments in the first place. Taking time to ensure you have the right fit is important. At the end of the day, you need to feel comfortable and confident that the people you choose can be counted on to deliver for you.
To me, one of the most valuable skills I look for with Informatics Consultants is the ability to bring a unique perspective. In a management training course I once took, the advisor summed it up this way: “Look, you are all smart people. If you come to a situation where you do not see a solution, it is probably because there is not just one solution. When you arrive at a situation like that you will have to find some way to balance and continually adjust, as there is likely no one right answer.” When I am bringing people in to support me on projects, I am looking for that ability to connect the dots and leverage previous experiences to help find the best solution.
One of the valuable parts of working as a consultant is that you get to see a variety of companies, all with different setups. While some people find that lifestyle stressful or challenging, there is an inherent value in being exposed to so many alternative organisational environments. If you can synthesize new information and learn rapidly, all these experiences add up to quite some knowledge. The more you can see things repeated with modified parameters, the easier you can find what works and what does not work, which is why we look to simulations to find some solutions. Informatics Consultants that have worked in different Biotech and big pharma settings with a wide exposure to different projects can help bring that knowledge to your organisation.
Finding consultants who have worked in various parts of life sciences or in other fields can also help to provide a more well-rounded view. At times, that enables seeing solutions in places you might not expect. They can also recognise patterns in the organisation you may be too close to see. The combination of being able to share these insights, as well having seen so many challenges and varying situations, can allow consultants to provide services to you that you simply are not able to manage internally.
Finding the right Informatics Consultant for your life sciences organisation is not an easy task; you need to make sure that you are bringing in the right skillset to match your situation.
The priority is to find people you can trust and who you feel confident can work in your environment. While the technology of course plays a role, do not overstate the importance of it and dismiss the (not insignificant) people and the processes aspect to our work. The system in itself will not be considered successful if people are not comfortably using it. Look for companies that can adjust to your needs and find solutions to your challenges as the landscape continues to change. In the end, that is what counts, finding a way to get the job done.
Scimcon is proud to offer its consultancy services to Biotech and big pharma companies around the world. To find out how we can help you achieve success in your implementation project, contact us.
The role of AI and ML in the future of lab informatics?User Acceptance Testing (UAT) is one of the latter stages of a software implementation project. UAT fits in the project timeline between the completion of configuration / customisation of the system and go live. Within a regulated lab or clinical setting UAT can be informal testing prior to validation, or more often forms the Performance Qualification (PQ).
Whether UAT is performed in a non-regulated or regulated environment it is important to note that UAT exists to ensure that business processes are correctly reflected within the software. In short, does the new software function correctly for your ways of working?
You would never go into any project without clear objectives, and software implementations are no exception. It is important to understand exactly how you need software workflows and processes to operate.
To clarify your needs, it is essential to have a set of requirements outlining the intended outcomes of the processes. How do you want each workflow to perform? How will you use this system? What functionality do you need and how do you need the results presented? These are all questions that must be considered before going ahead with a software implementation project.
Creating detailed requirements will highlight areas of the business processes that will need to be tested within the software by the team leading the User Acceptance Testing.
Requirements, like the applications they describe, have a lifecycle and they are normally defined early in the purchase phase of a project. These ‘pre-purchase’ requirements will be product independent and will evolve multiple times as the application is selected, and implementation decisions are made.
While it is good practice to constantly revise the requirements list as the project proceeds, it is often the case that they are not well maintained. This can be due to a variety of reasons, but regardless of the reason you should ensure the system requirements are up to date before designing your plan for UAT.
A common mistake for inexperienced testing teams is to test too many items or outcomes. It may seem like a good idea to test as much as possible, but this invariably means all requirements from critical to the inconsequential are tested to the same low level.
Requirements are often prioritised during product selection and implementation phases according to MoSCoW analysis. This divides requirements into Must-have, Should-have, Could-have and Wont-have and is a great tool for assessing requirements in these earlier phases.
During the UAT phase these classifications are less useful, for example there may be requirements for a complex calculation within a LIMS, ELN or ePRO system. These calculations may be classified as ‘Could-have’ or low priority because there are other options to perform the calculations outside of the system. However, if these calculations are added to the system during implementation, they are most likely, due to their complexity, a high priority for testing.
To avoid this the requirements, or more precisely their priorities, need to be re-assessed as part of the initial UAT phase.
A simple but effective way to set priority is to assess each requirement against the risk criteria and assign a testing score. The following criteria are often used together to assess risk:
Once the priority of the requirements has been classified the UAT team can then agree how to address the requirements in each category.
A low score could mean the requirement is not tested or included in a simple checklist.
A medium score could mean the requirement is included in a test script with several requirements.
A high score could mean the requirement is the subject of a dedicated test script.
A key question often asked of our team is how many test scripts will be needed and in what order should they be executed? These questions can be answered by creating a Critical Test Plan (CTP). The CTP approach requires that you first rise above the requirements and identify the key business workflows you are replicating in the system. For a LIMS system these would include:
Sample creation, Sample Receipt, Sample Prep, Testing, Result Review, Approval and Final Reporting.

Next the test titles required for each key workflow are added in a logical order to a CTP diagram, which assists in clarifying the relationship between each test. The CTP is also a great tool to communicate the planned testing and helps to visualise any workflows that may have been overlooked.
Now that the test titles have been decided upon, requirements can be assigned to a test title and we are ready to start authoring the scripts.
There are several different approaches to test script formats. These range from simple checklists, ‘objective based’ where an overview of the areas to test are given but not the specifics of how to test, to very prescriptive step by step instruction-based scripts.
When testing a system within the regulated space you generally have little choice but to use the step by step approach.
Test scripts containing step by step instruction should have a number of elements for each step:
A typical example is given below.
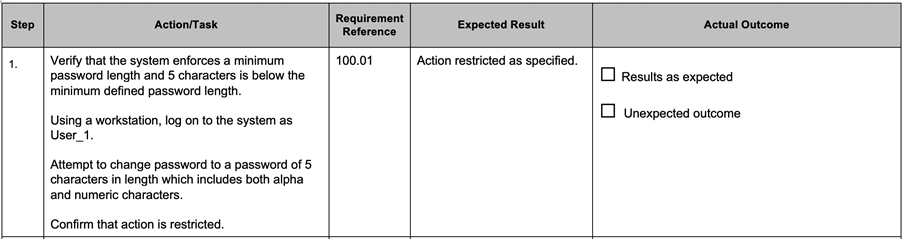
However, when using the step by step format for test scripts, there are still pragmatic steps that can be taken to ensure efficient testing.
Data Setup – Often it is necessary to create system objects to test within a script. In an ELN this could be an experiment, reagent or instrument, or in ePRO a subject or site. If you are not directly testing the creation of these system objects in the test script, their creation should be detailed in a separate data setup section outside of the ‘step by step’ instructions. Besides saving time during script writing, any mistakes made in the data setup will not be classified as script errors and can be quickly corrected without impacting test execution.
Low Risk Requirements – If you have decided to test low risk requirements then consider the most appropriate way to demonstrate that they are functioning correctly. A method we have used successfully is to add low risk requirements to a table outside of the step by step instructions. The table acts as a checklist with script executers marking each requirement that they see working correctly during executing the main body of step by step instructions. This avoids adding the low requirements into the main body of the test script but still ensures they are tested.
Test Script Length – A common mistake made during script writing is to make them too long. If a step fails while executing a script, one of the resulting actions could be to re-run the script. This is onerous enough when you are on page 14 of a 15 page script. However, this is significantly more time-consuming if you are on page 99 out of 100. While there is no hard and fast rule on number of steps or pages to have within a script, it is best to keep them to a reasonable length. An alternative way to deal with longer scripts is to separate them into sections which allows the option of restarting the current block of instructions within a script, instead of the whole script.
An important task when co-ordinating UAT is be fully transparent about which requirements are to be tested and in which scripts. We recommend adding this detail against each requirement in the User Requirements Specification (URS). This appended URS is often referred to as a Requirements Trace Matrix. For additional clarity we normally add a section to each test script that details all the requirements tested in the script as well as adding the individual requirement identifiers to the steps in the scripts that test them.
UAT is an essential phase in implementing new software, and for inexperienced users it can become time-consuming and difficult to progress. However, following the above steps from our team of experts will assist in authoring appropriate test scripts and leading to the overall success of a UAT project. In a future blog we will look at dry running scripts and formal test execution, so keep an eye on our Opinion page for further updates.
Digital transformation: Revolutionising the labs of the future?